Leukodystrophies Overview
Leukodystrophies, which have an incidence of > 1 in 5,000 live births, affect nervous system myelin (Bonkowsky, 2010). Most leukodystrophies lack treatment and have >30% mortality by 8 years of age. Leukodystrophies are relatively rare, leading to a limited understanding of disease pathophysiology.
Our Lab uses zebrafish as a tool to study leukodystrophies. Zebrafish are a genetically tractable organism that are transparent as embryos, allowing for fluorescent biological marker expression. Furthermore, zebrafish produce large numbers of offspring with a relatively short maturation. We also have mammalian models to further explore and apply relevant findings from our zebrafish studies. Our goal is to screen compounds and gene therapies for prevention or cure for the various leukodystrophies that are so devastating to patients and families.
Pictured: Olig2dsRed-stained zebrafish heart
Vanishing White Matter (VWM) Disease
Vanishing White Matter (VWM) Disease is a rare autosomal recessive form of leukodystrophy, caused by mutations in the eukaryotic initiation factor 2B (eIF2B) complex. Mutations in any of the five subunits encompassing the eIF2B complex may lead to VWM disease, causing neurodegeneration from central nervous system (CNS) demyelination, and high rates of morbidity and mortality. We have developed and are characterizing a zebrafish model of VMW disease. We have generated mutant alleles in zebrafish eIF2B subunits, and these animals exhibit a range of phenotypic severity, including changes in growth, lethality, behavior, myelination, apoptosis, and proliferation in the CNS. We also see a conservation of function between the eif2B-2 subunit of zebrafish and human models. Eif2B mutants at baseline have an intrinsically activated integrated stress response (ISR), and we are currently exploring this pathway in zebrafish mutants and in a mouse model of VWM disease.
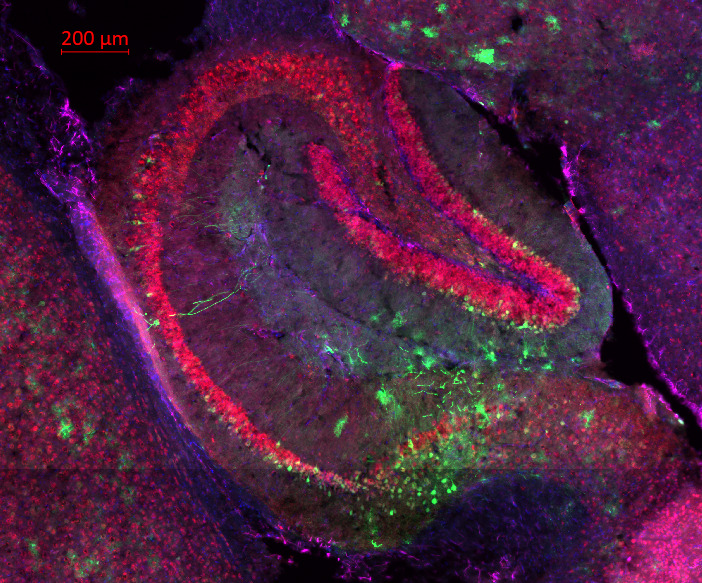
Pictured: Multi-Stained (DAPI, GFAP, GFP, NeuN) mouse hippocampus in VWM disease model
X-Linked Adrenoleukodystrophy (X-ALD)
X-ALD is a severe metabolic and neurodegenerative leukodystrophy. This disorder is caused by mutations in the ABCD1 gene, which encodes a peroxisomal transporter involved in the degradation of very long chain fatty acids (VLCFA). X-ALD is characterized by phenotypic heterogeneity, resulting in a delayed diagnosis in the absence of newborn screening and remains without any satisfying therapy or disease models. An ABCD1 mutant zebrafish model has been generated in our lab and it phenocopies several traits found in patients; there is an accumulation of VLCFA, adrenal-like insufficiency, increased oligodendrocyte apoptosis, and reduced survival and swimming behavior. Our work is now focusing in on characterizing the relation between the peroxisomal defect and the myelin disruption in X-ALD. Taking advantage of the zebrafish high fecundity and rapid development, we are screening drug candidates capable of improving myelin restoration in X-ALD. (We are also interested in other peroxisomal leukodystrophies and investigating the potential benefit of gene therapies for these diseases.)
Stress Granules and the Integrated Stress Response (ISR)
Neurodegenerative diseases including Leukodystrophy with Vanishing White Matter (VWM) have activation of the integrated stress response (ISR). Our objective is analysis of stress granules (SGs), membrane-less cytoplasmic ribonucleoprotein aggregations, which have been identified as a key mediator of ISR activation and pathophysiology. We have found an increase in SGs and in apoptosis in the brains of zebrafish and mice with VWM; more severe mutants had larger increases in SGs and apoptosis. Our results suggest that SGs mediate VWM disease pathophysiology, and that staufen1 acts as a feedback link between ISR activation and SG formation. Our findings suggest novel opportunities to disrupt CNS pathophysiology of VWM. Further, there may be mechanisms of neurodegeneration shared from VWM with more common adult neurodegenerative conditions.
Pictured: Zebrafish stained with TIAL1, DAPI, and ZRF
Pelizaeus-Merzbacher Disease (PMD)
PMD is caused by mutations in the gene PLP1, which encodes a major protein of the myelin sheath, proteolipid protein. Over half of PMD cases are caused by a complete duplication of the PLP1 gene. We are currently developing a zebrafish model of PMD that can then be used to further characterize the disease and research treatments.